Influenza ( 2009 H1N1 )
Scientific & Medical Resources
Swine Flu, Influenza A, 2009 H1N1 , Pandemic, Medical, Scientific, links, medical journals, sites, videos, RSS feeds, original research papers
This knol has been created to provide a single source of all the medical and scientific advances with live RSS feeds, Video and direct links to all the original papers and biomedical advances in the fight against the Pandemic.
Table des matières
- Sponsorship, Commercial Use and IPR
- Influenza A ( 2009 H1N1 ) Pandemic FDA Advisory
- Influenza A ( 2009 H1N1 ) Pandemic Official Alerts, Advisory
- Be Healthy! Visit www.cdc.gov Centers...
- Medical News Resources
- PLoS Currents - Research Knols Explained
- Consensus statement from an inter-agency scientific consultation on potential risks of pandemic (H1N1) 2009 influenza virus at the human-animal...
- Books and Monographs
- Influenza
- Results: 1 to 20 of 1344
- Results: 1 to 20 of 1347
- Acknowledgements
Search only trustworthy HONcode health websites:
Publishers, corporations, educational, academic, government, foundations, organizations and readers are invited to read the following document for funding, sponsorship and commercial license opportunities.
The document explains author's policy: to provide trustworthy information, conflict of interest, marketplace and privacy and data protection issues.
LES AUTEURS

Krishan Maggon
Consultant Pharmaceutical Biotechnology R&D & Advisor
Geneva, Switzerland & New York, USA
Professor Medical Analyses and Medical bacteriology / Scientific Author / knolAuteur

Serving the publishing aspirations of Knol writers, Planet Earth
CDC Transmission Electron Micrograph of H1N1 Virus
CDC PHIL 00528
References, sources and updates:
All the articles, alerts and RSS feeds google, dealing with Swine Flu [Influenza A (H1N1)v] Pandemic and Reviews
Influenza A ( 2009 H1N1 ) Pandemic FDA Advisory
Consumers are strongly urged and warned not to buy or order any medicine or vaccine from web and internet sites.
About 50 websites were warned by the FDA to remove or delete unapproved products to diagnose,treat or prevent H1N1 Influenza or Swine Flu. The products with false claims included shampoo, dietary supplements, spray with ionic silver, gloves, unapproved diagnostic tests and electronic energy device. These products were considered illegal by the FDA and are a significant threat to public health.


Talk about organic content!
Click here to try Google's latest
tool on the topic of Swine Flu.
Very COOL timeline, too.
![[Ways to produce a flu vaccine chart]](http://s.wsj.net/public/resources/images/NA-BC268A_VACCI_NS_20091124215328.gif)
WHO.
Clinical management of human infection with new influenza A (H1N1) virus: initial guidance. www.who.int/csr/.../clinical_managementH1N1_21_May_2009.pdf
Influenza A ( 2009 H1N1 ) Pandemic Official Alerts, Advisory
Multilingual
WHO in Arabic, Chinese, English, French, Russian, Spanish

Recommended use of antivirals
21 August 2009
Call to action
17 August 2009
Situation updates
(Last update 21 August 2009)
21 August 2009
Call to action
17 August 2009
Situation updates
(Last update 21 August 2009)
http://ec.europa.eu/health/ph_threats/com/Influenza/novelflu_de.htm
MEMO/09/362
MEMO/09/363
MEMO/09/362
MEMO/09/363
EMEA European Medicines Agency
Health Canada English, French
Switzerland French, German, Italian, English
UK
France
USA
CDC, English, Spanish


CDC Advisors Make Recommendations for Use of Vaccine Against Novel H1N1. http://www.cdc.gov/media/pressrel/2009/r090729b.htm
2009 H1N1 Flu
Site last updated December 28, 2:00 PM ETSituation Update
The weekly activity update is being provided one day early due to the Federal Holiday on Friday.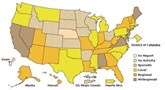 Flu activity continued to decline in the United States during the week of December 13-19, 2009, as reported in FluView. The number of states reporting widespread flu activity decreased from 11 to 7. Visits to doctors for influenza-like illness, flu-associated hospitalizations, and flu-associated deaths all declined from the previous week. Flu is unpredictable and activity can rise and fall throughout the season, but flu is is likely to continue for months caused by either 2009 H1N1 viruses or regular seasonal flu viruses. In addition to seasonal flu vaccine, a vaccine against the 2009 H1N1 virus has been produced and is the best way to protect against the pandemic virus. Supplies of this vaccine are increasing and many places have opened up vaccination to anyone who wants it. Find a vaccine.
Flu activity continued to decline in the United States during the week of December 13-19, 2009, as reported in FluView. The number of states reporting widespread flu activity decreased from 11 to 7. Visits to doctors for influenza-like illness, flu-associated hospitalizations, and flu-associated deaths all declined from the previous week. Flu is unpredictable and activity can rise and fall throughout the season, but flu is is likely to continue for months caused by either 2009 H1N1 viruses or regular seasonal flu viruses. In addition to seasonal flu vaccine, a vaccine against the 2009 H1N1 virus has been produced and is the best way to protect against the pandemic virus. Supplies of this vaccine are increasing and many places have opened up vaccination to anyone who wants it. Find a vaccine.Other 2009 H1N1 Flu Topics
Diagnosis
How the illness is diagnosed, recommendations for lab testing…Infection Control
Healthcare guidance, occupational safety, facemasks & respirators…Antivirals/Treatment
Use of Tamiflu and Relenza for treatment or prevention of H1N1 flu…Emergency Use Authorization
Info about CDC-requested & FDA-issued EUA drugs & devices…
What You Can Do to Stay Healthy
- Get vaccinated. Vaccination is the best protection we have against flu.Seasonal flu vaccine is available now and initial doses of 2009 H1N1 flu vaccine also are available, with additional doses available later this year.
- Influenza is thought to spread mainly person-to-person through coughing or sneezing of infected people.
- Take everyday actions to stay healthy.
- Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Wash your hands often with soap and water. If soap and water are not available, use an alcohol-based hand rub.
- Avoid touching your eyes, nose and mouth. Germs spread that way.
- Stay home if you get sick. CDC recommends that you stay home from work or school and limit contact with others to keep from infecting them.
- Follow public health advice regarding school closures, avoiding crowds and other social distancing measures.
- Stay informed. This website will be updated regularly as information becomes available.
- Call 1-800-CDC-INFO for more information.
- Email page
- Print page
- Bookmark and share
- Add this to...
- Favorites
- Del.icio.us
- Digg
- Facebook
- Google Bookmarks
- Yahoo MyWeb
View page in
Get email updates
To receive weekly email updates about this site, enter your email address:What's this?Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333
- 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348
24 Hours/Every Day
- cdcinfo@cdc.gov
Spread the word
Site last updated December 28, 2:00 PM ET
Situation Update
The weekly activity update is being provided one day early due to the Federal Holiday on Friday.
 Flu activity continued to decline in the United States during the week of December 13-19, 2009, as reported in FluView. The number of states reporting widespread flu activity decreased from 11 to 7. Visits to doctors for influenza-like illness, flu-associated hospitalizations, and flu-associated deaths all declined from the previous week. Flu is unpredictable and activity can rise and fall throughout the season, but flu is is likely to continue for months caused by either 2009 H1N1 viruses or regular seasonal flu viruses. In addition to seasonal flu vaccine, a vaccine against the 2009 H1N1 virus has been produced and is the best way to protect against the pandemic virus. Supplies of this vaccine are increasing and many places have opened up vaccination to anyone who wants it. Find a vaccine.
Flu activity continued to decline in the United States during the week of December 13-19, 2009, as reported in FluView. The number of states reporting widespread flu activity decreased from 11 to 7. Visits to doctors for influenza-like illness, flu-associated hospitalizations, and flu-associated deaths all declined from the previous week. Flu is unpredictable and activity can rise and fall throughout the season, but flu is is likely to continue for months caused by either 2009 H1N1 viruses or regular seasonal flu viruses. In addition to seasonal flu vaccine, a vaccine against the 2009 H1N1 virus has been produced and is the best way to protect against the pandemic virus. Supplies of this vaccine are increasing and many places have opened up vaccination to anyone who wants it. Find a vaccine.Other 2009 H1N1 Flu Topics
Diagnosis
How the illness is diagnosed, recommendations for lab testing…
Infection Control
Healthcare guidance, occupational safety, facemasks & respirators…
Antivirals/Treatment
Use of Tamiflu and Relenza for treatment or prevention of H1N1 flu…
Emergency Use Authorization
Info about CDC-requested & FDA-issued EUA drugs & devices…
What You Can Do to Stay Healthy
- Get vaccinated. Vaccination is the best protection we have against flu.Seasonal flu vaccine is available now and initial doses of 2009 H1N1 flu vaccine also are available, with additional doses available later this year.
- Influenza is thought to spread mainly person-to-person through coughing or sneezing of infected people.
- Take everyday actions to stay healthy.
- Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Wash your hands often with soap and water. If soap and water are not available, use an alcohol-based hand rub.
- Avoid touching your eyes, nose and mouth. Germs spread that way.
- Stay home if you get sick. CDC recommends that you stay home from work or school and limit contact with others to keep from infecting them.
- Follow public health advice regarding school closures, avoiding crowds and other social distancing measures.
- Stay informed. This website will be updated regularly as information becomes available.
- Call 1-800-CDC-INFO for more information.
- Email page
- Print page
- Bookmark and share
- Add this to...
- Favorites
- Del.icio.us
- Digg
- Google Bookmarks
- Yahoo MyWeb
View page in
Get email updates
To receive weekly email updates about this site, enter your email address:
What's this?Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348
24 Hours/Every Day - cdcinfo@cdc.gov
Spread the word
Site last updated December 11, 3:00 PM ET
Dec 1 ALERT: Fraudulent emails referencing CDC-sponsored State Vaccination Program »
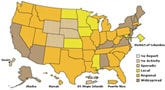 During the week of November 29-December 5, 2009, flu activity continued to decline in the United States as reported in FluView. The number of states reporting widespread flu activity decreased from 25 to 14. Visits to doctors for influenza-like illness and flu-associated hospitalizations declined from the previous week, however flu-associated deaths increased.
During the week of November 29-December 5, 2009, flu activity continued to decline in the United States as reported in FluView. The number of states reporting widespread flu activity decreased from 25 to 14. Visits to doctors for influenza-like illness and flu-associated hospitalizations declined from the previous week, however flu-associated deaths increased.
See More On Key Flu Indicators »
Situation Update
 During the week of November 29-December 5, 2009, flu activity continued to decline in the United States as reported in FluView. The number of states reporting widespread flu activity decreased from 25 to 14. Visits to doctors for influenza-like illness and flu-associated hospitalizations declined from the previous week, however flu-associated deaths increased.
During the week of November 29-December 5, 2009, flu activity continued to decline in the United States as reported in FluView. The number of states reporting widespread flu activity decreased from 25 to 14. Visits to doctors for influenza-like illness and flu-associated hospitalizations declined from the previous week, however flu-associated deaths increased. See More On Key Flu Indicators »
Other 2009 H1N1 Flu Topics
Diagnosis
How the illness is diagnosed, recommendations for lab testing…Infection Control
Healthcare guidance, occupational safety, facemasks & respirators…Antivirals/Treatment
Use of Tamiflu and Relenza for treatment or prevention of H1N1 flu…Emergency Use Authorization
Info about CDC-requested & FDA-issued EUA drugs & devices…What You Can Do to Stay Healthy
- Get vaccinated. Vaccination is the best protection we have against flu. Seasonal flu vaccine is available now and initial doses of 2009 H1N1 flu vaccine also are available, with additional doses available later this year.
- Influenza is thought to spread mainly person-to-person through coughing or sneezing of infected people.
- Take everyday actions to stay healthy.
- Cover your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Wash your hands often with soap and water. If soap and water are not available, use an alcohol-based hand rub.
- Avoid touching your eyes, nose and mouth. Germs spread that way.
- Stay home if you get sick. CDC recommends that you stay home from work or school and limit contact with others to keep from infecting them.
- Follow public health advice regarding school closures, avoiding crowds and other social distancing measures.
- Stay informed. This website will be updated regularly as information becomes available.
- Call 1-800-CDC-INFO for more information.
- Email page
- Print page
- Bookmark and share
- Add this to...
- Favorites
- Del.icio.us
- Digg
- Google Bookmarks
- Yahoo MyWeb
View page in
Get email updates
To receive daily email updates about this site, enter your email address:Enter Email Address Submit Button What's this?
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348
24 Hours/Every Day - cdcinfo@cdc.gov
Swine Flu Info
Quick Question
Are these search results helpful to you? Why or why not?
Thank you for helping us improve our site.
Spread the word
FDA 2009 H1N1 (Swine) Flu Page
The FDA plays a vital role on the team led by the U.S. Department of Health and Human Services in the fight against the 2009 H1N1 virus. The agency works closely with the Centers for Disease Control and Prevention, National Institutes of Health, other federal government agencies, and global partners such as the World Health Organization and foreign governments to protect public health during the 2009 H1N1 pandemic. Topics on this Page: |
H1N1 Flu Vaccine
FDA ensures the safety, effectiveness and supply of the H1N1 flu vaccine.- How the flu vaccine is made (PDF, 248 KB)
- H1N1 vaccine ingredients
- H1N1 vaccine questions and answers
- Use of Influenza A (H1N1) 2009 Monovalent Influenza Vaccine in Pregnant Women
- Dear health care professionals letter regarding 2009 H1N1 flu virus (June 18)
- Complete information on each H1N1 flu vaccine
- Injectable vaccine
- Intranasal vaccine
- Safety monitoring
- FDA update on the H1N1 flu vaccine and antiviral medications (no date)
- News Releases
- FDA Approves Additional Vaccine for 2009 H1N1 Influenza Virus (November 16)
- FDA Expands Approved Use of H1N1 Vaccines to Include Infants and Children (November 12)
- FDA Commissioner Addresses Nation’s Healthcare Professionals on H1N1 Vaccine (November 10)
- FDA Approves Vaccines for 2009 H1N1 Influenza Virus (September 15)
- FDA Approves New Influenza Vaccine Production Facility (May 6)
Consumer Protection: Fraudulent H1N1 Claims and Bogus H1N1 Products
FDA tracks and prosecutes advertisers who make fraudulent claims in ads about preventing or treating the H1N1 virus.Antiviral Drugs
FDA ensures the safety, effectiveness and supply of flu antiviral drugs.- Drug Treatments for Flu (antivirals)
- Guidance to Pharmacies on Advance Compounding of Tamiflu Oral Suspension to Provide for Multiple Prescriptions (October 31)
- Updated Questions and Answers: 2009 H1N1 Flu Virus and Emergency Use Authorization of Tamiflu and Relenza (October 31)
- Peramivir IV Questions and Answers for Health Care Providers
- Q&A for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV (PDF, 22 KB)
- Pregnancy and antiviral use
- Tamiflu Oral Suspension Shortage Information
- Stockpiled Antivirals at or Nearing Expiration
- FDA public health alert: potential medication errors with Tamiflu for oral suspension (September 25)
- Information for healthcare professionals: authorization of use of expired Tamiflu for oral suspension (October 2)
- Dear health care professionals letter regarding 2009 H1N1 flu virus (June 18)
- FDA update on the H1N1 flu vaccine and antiviral medications (no date)
- Emergency use authorizations
- Emergency use authorizations questions and answers (April 28)
- FDA and CDC Information on Potential “Spot Shortages” of Supplies for Treating and Preventing Novel Influenza A (H1N1) (June 18)
- News Releases
Diagnostic Tests
FDA approves diagnostic tests for laboratories, clinics and doctor’s offices.- Medical devices for flu diagnosis and protection (October 16)
- Dear health care professionals letter regarding 2009 H1N1 flu virus (June 18)
- Health care professional fact sheet: interpreting swine influenza RT-PCR detection panel test results (May 2)
- Patient fact sheet: understanding swine flu kit test results (May 2009)
- Emergency Use Authorizations for Devices (April 28)
- News Releases
Safety of the Food Supply
FDA ensures the safety of the food supply.Back to Top
Safety of the Blood Supply
Back to TopSupply of Masks, Gloves and Other Personal Protective Equipment
FDA monitors the supply of facemasks, respirators and other personal protective equipment.- Personal protective equipment – facemasks, respirators, etc. general information
- Questions and answers
- FDA and personal protective equipment for the 2009 H1N1 flu virus (September 15)
 |  |
-
-
Page Last Updated: 12/09/2009
Note: If you need help accessing information in different file formats, see Instructions for Downloading Viewers and Players.
Note: If you need help accessing information in different file formats, see Instructions for Downloading Viewers and Players.
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||
| ||||||||||||||












 Send an e-card
Send an e-card
 Send an e-card
Send an e-card
















![Swine Flu [Influenza A (H1N1)v] Pandemic Review Swine Flu [I...](http://knol.google.com/k/-/-/1yexqll01wr4o/9ko4nj/11215lores1.jpg?width=48&height=48)








Aucun commentaire:
Enregistrer un commentaire